Notch program
Notch is a major developmental pathway that regulates cancer stem cells (CSCs) in Notch-driven cancers, such as certain types of T-cell lymphomas and esophageal adenocarcinomas (EACs). Notch signaling is initiated upon the physical interaction of cells expressing ligands with neighboring cells expressing Notch receptors (Notch1-4). Notch ligand/receptor interaction results in irreversible cleavage of Notch receptors by gamma-secretase and subsequent generation of Notch intracellular domains (NICDs). NICDs translocate to the nucleus and are required for the stepwise formation of an active Notch Transcription Complex (NTC) that includes recruitment of the DNA-binding protein CSL (C promoter-binding factor-1, Suppressor of Hairless, Lag1), followed by the transcriptional coactivator Mastermind-like 1 (Maml1). The NTC subsequently recruits additional coactivators (CoA) through the Maml1 C-terminal domain and drives transcription of target genes. Therefore, approaches that prevent NTC assembly will inhibit NICDs-directed transcription, thus abolishing the growth of Notch-driven cancers.
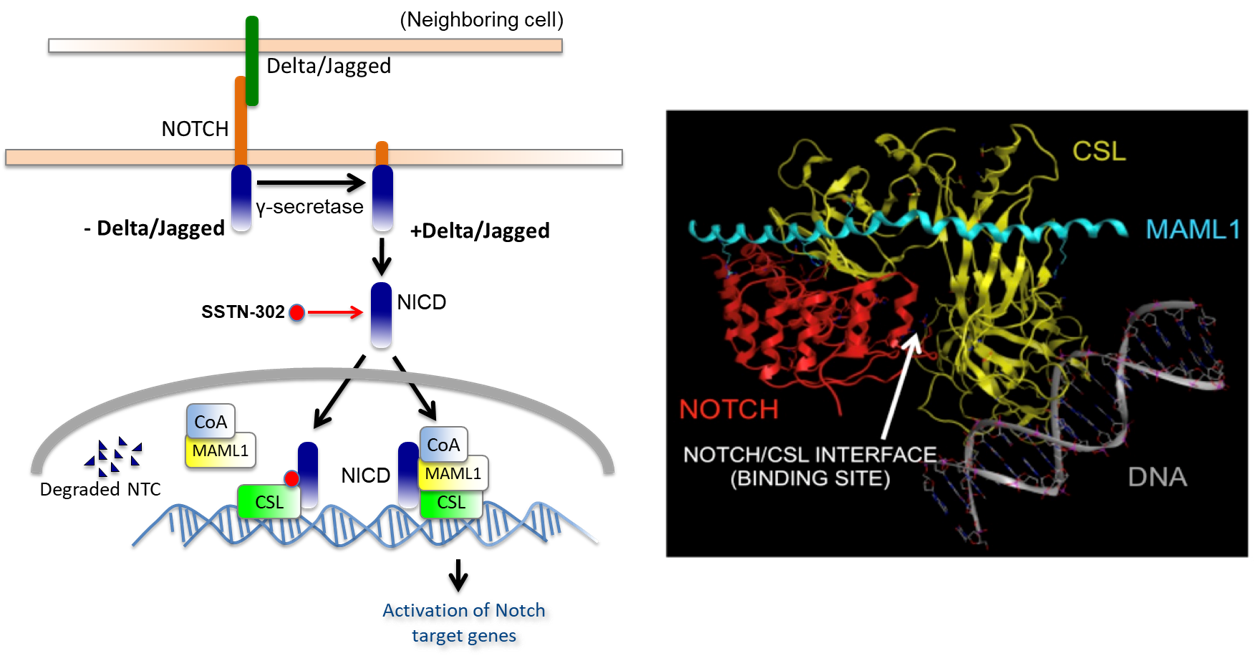
SSTI have identified a targetable small molecule binding site within the NTC that represents a unique “druggable” target downstream of Notch1 intracellular domain (Notch1ICD). Our lead compound, SSTN-302, functions to specifically disrupt Notch1-mediated NTC formation.
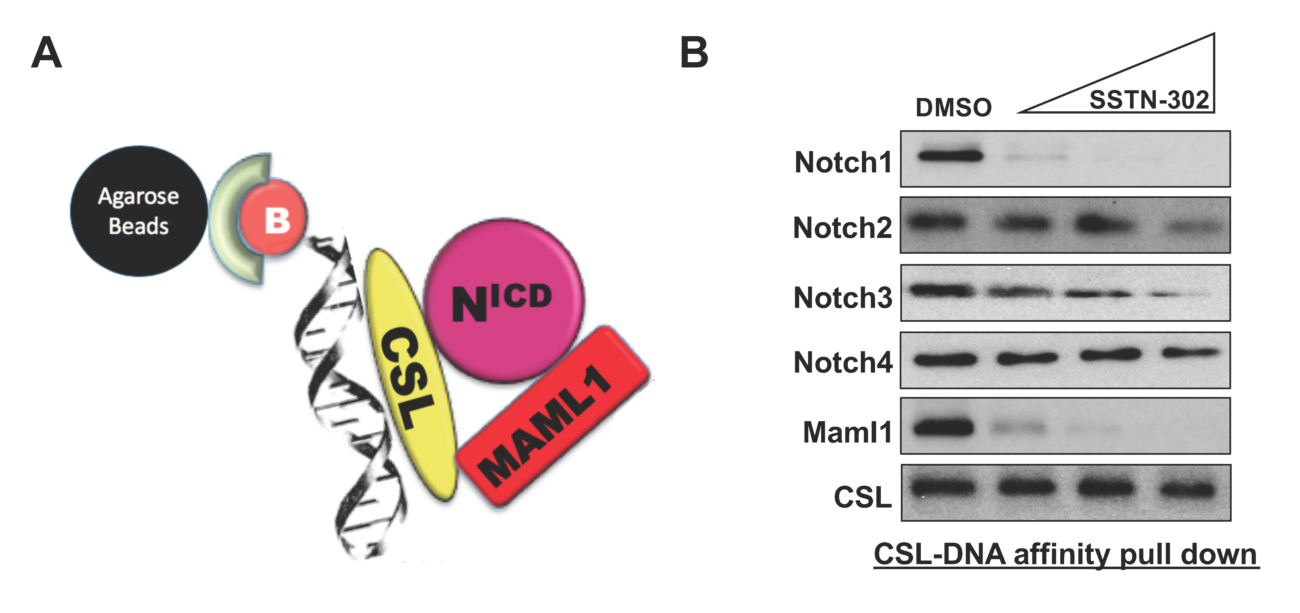
SSTN-302 is a Notch1-specificity inhibitor. (A) A cartoon description of the CSL-DNA affinity pull down assay. (B) SSTN-302 specifically disrupts Notch1-mediated NTC formation. Human breast cancer cells were treated with vehicle (DMSO) or escalating doses of SSTN-302. The nuclear extracts of the treated cells contained equal protein levels of the NTC components (data not shown), and were used for the CSL-DNA affinity pull down assay. The proteins were then quantified by immunoblots.
SST-302 and analogs are first in-class Notch pathway inhibitors:
- A novel mechanism of action and cellular target
- Specific to Notch1-mediated Notch signaling
- Potent inhibitors of Notch signaling
- Highly bioavailable
- Efficacious in eradicating tumor growth in cell culture and in vivo
- No gastrointestinal tract toxicity that is typically associated with pan-Notch inhibition
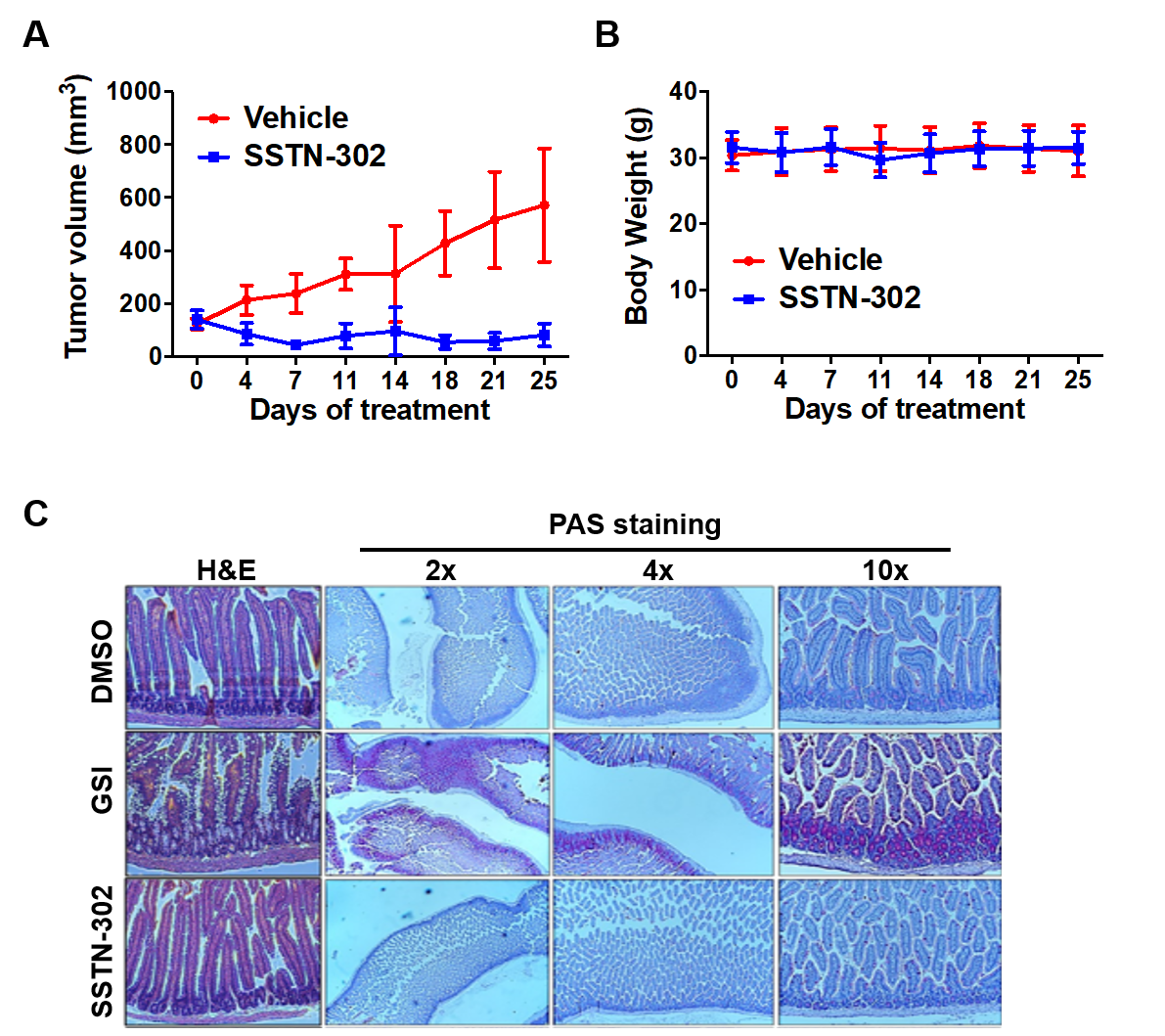
SSTN-302 inhibits tumor growth in vivo. SSTN-302 inhibits the growth of PC-3 xenografts (A) in mice without apparent toxicity (B). (C) SSTN-302 treatment does not cause goblet cell metaplasia in mice. Wild type mice were treated with SSTN-302 or a gamma-secretase inhibitor (GSI) for five days. Pan-Notch inhibition by GSI causes goblet cell metaplasia as reported previously.