Familial Adenomatous Polyposis program
Individuals with the inherited disorder Familial Adenomatous Polyposis (FAP) develop thousands of precancerous polyps at an early age that ultimately develop into colorectal cancer (CRC), if untreated. The current standard of care for FAP patients is the complete removal of the colon (colectomy) during their teenage years.
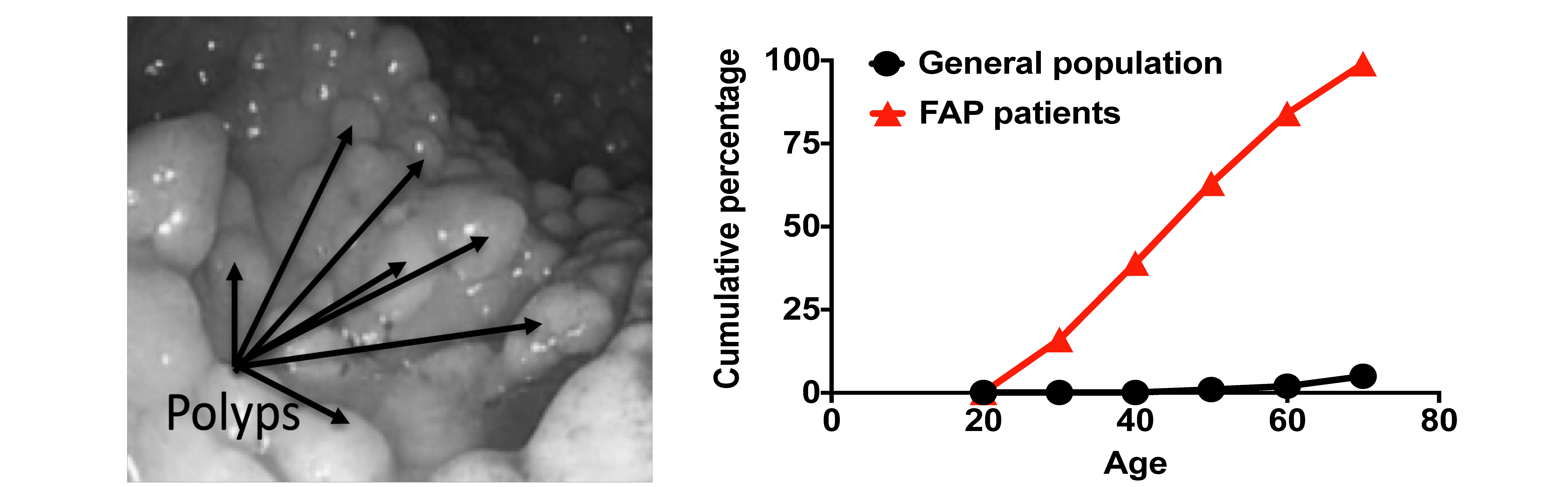 Polyp presentation, and incidence of colorectal cancer by age in FAP.
Polyp presentation, and incidence of colorectal cancer by age in FAP.
Left panel: A representative colonoscopy image from a 17-year-old FAP patient (image credit: medbullets.com). Right panel: Cumulative incidence of colorectal cancer by age in FAP patients compared with the general population (Winawer, S.J., et al., Gastroenterology, 1997).
FAP is driven almost exclusively by mutational activation of Wnt signaling (mautations in the APC gene). However, no Wnt inhibitors have been clinically approved to treat FAP patients. We identified Pyrvinium (SST-024), an off-patent FDA approved pinworm drug, as a potent Wnt inhibitor.
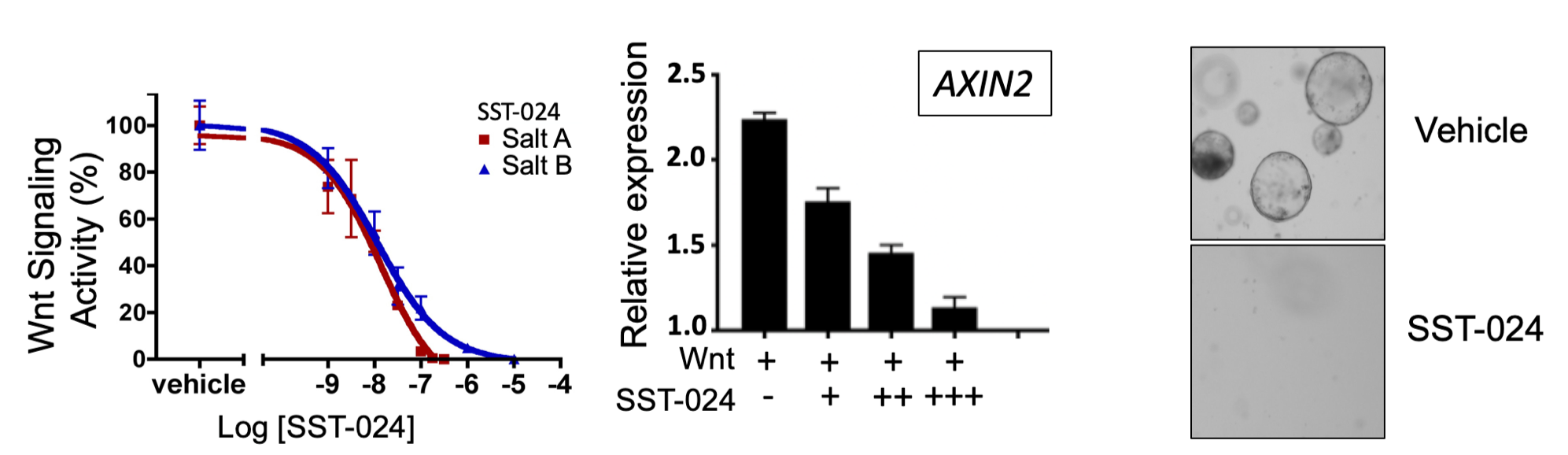 A large-scale compound screen identified SST-024 as a potent Wnt inhibitor.
A large-scale compound screen identified SST-024 as a potent Wnt inhibitor.
SST-024 inhibits Wnt signaling reporter activity (left panel), the expression of the Wnt signaling target gene AXIN2 (middle panel) and abrogates the growth of three-dimensional APC mutant intestinal organoids (right panel).
APCmin is a genetically engineered mouse model that mimics the mutation found in FAP patients. These mice develop intestinal polyps that are similar to those found in FAP patients. SST-024 treatment attenuates the growth of intestinal polyps in these mice and does so in a manner that does not display the on-target gut toxicity that has hampered the development of Wnt inhibitors. The efficacious dose used in these mice is less than 50% of the standard dose used in pinworm patients.
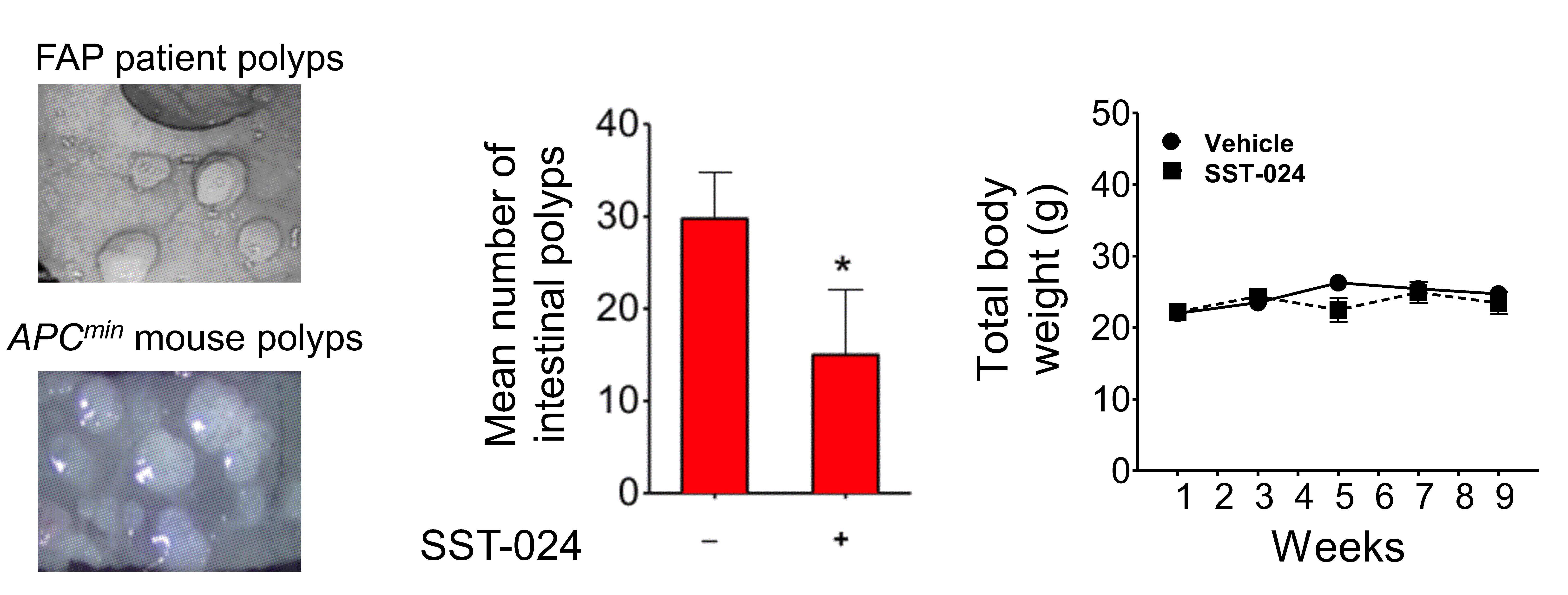 SST-024 attenuates the growth of intestinal polyps in a FAP mouse model.
SST-024 attenuates the growth of intestinal polyps in a FAP mouse model.
The APCmin mouse model recapitulates the disease of human FAP (left panel, image credit: gastrointestinalatlas.com and World Journal of Gastroenterology). SST-024 treatment decreased the polyp formation in these mice (middle panel) without demonstrating toxicity (right panel).
SSTI was awarded an Orphan Drug Designation by the FDA to develop SST-024 as a targeted therapeutic agent for FAP patients. As SST-024 had been previously FDA-approved as an anti-pinworm medication, its repurposing for the treatment of FAP represents a rapid, mechanistically relevant approach to address this urgent clinical need.
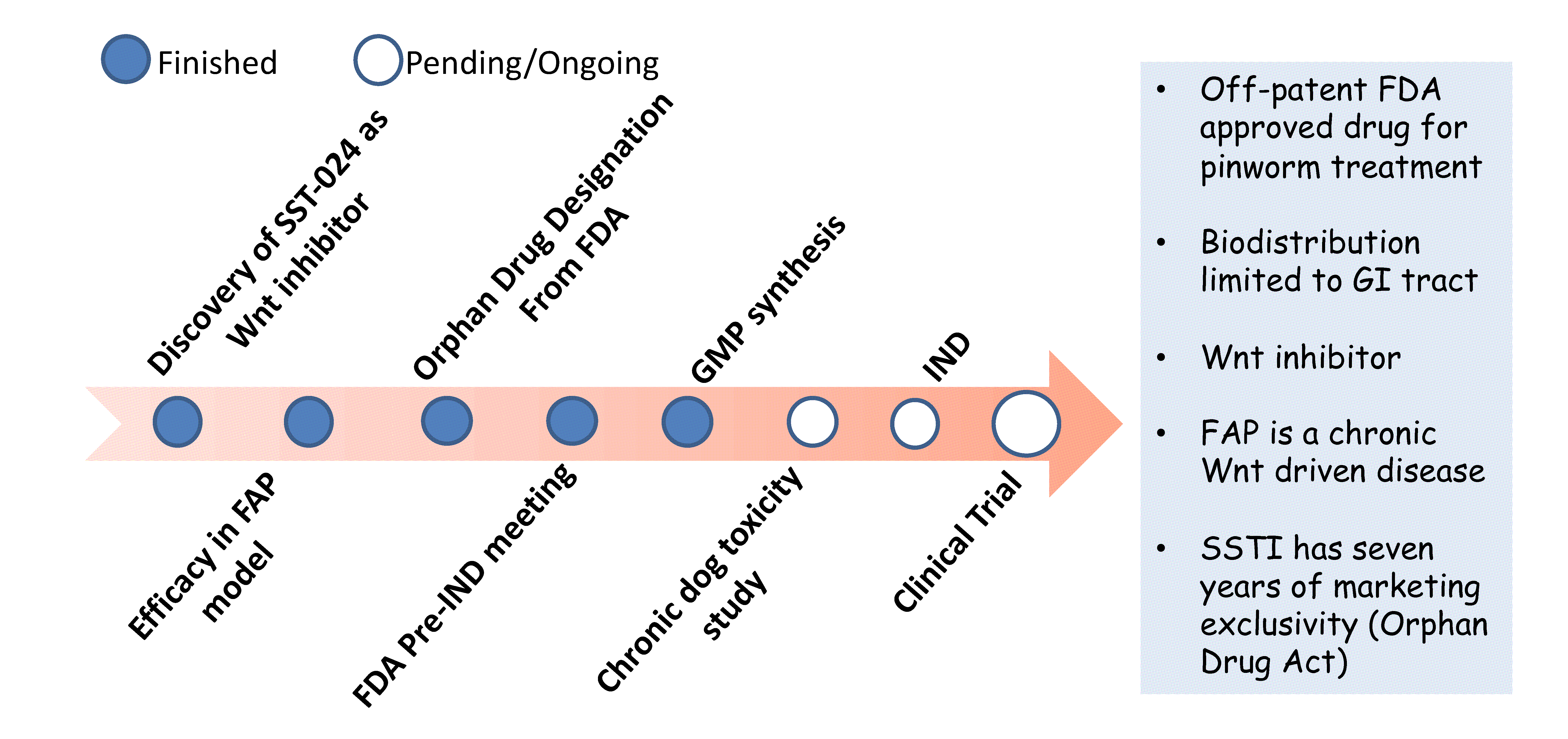 Milestones in the repurposing of SST-024 to treat FAP patients
Milestones in the repurposing of SST-024 to treat FAP patients
When FDA approved, SST-024 would represent:
- A major improvement in the treatment and quality of life of FAP patients.
- A successful bench to bedside application of a rationally selected targeted therapy.